What Is The Electron Geometry Of Bcl3
Chemistry questions and answers. Bond angle of octahedral.

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
How do I determine the bond angle in a molecule.

What is the electron geometry of bcl3. Predicting molecular geometry To determine the molecular geometry Find number of valence electrons Draw the Lewis structure Count the number of electron pairs bond pairs and lone pairs but count multiple bonds as one pair Arrange electron pairs to minimise repulsion Name the geometry from the atom positions. According to VSEPR theory the molecular geometry of boron trichloride is trigonal planar with a bond angle of 120 degrees. Boron forms 3 sp-p bonds with three chlorine atoms.
How many o and bonds are there. Note that Boron can have a full outershell with only six valence electrons. B egtetrahedral mgtrigonal planar.
We can see from the chart that BCl3 is an AX3 type molecule. Our mission is to help you succeed in your Chemistry class. Clutch really helped me by reinforcing the.
Bond angle of linear. It has a tetrahedral electron geometry and trigonal pyramidal shape. Boron trichloride or BCl3 is nonpolar.
What is the hybridization of the central atom. The three chloride atoms have a negative charge and the one boron in the center has an equal but positive charge. I hope that this blog post helps you understand all the aspects of this molecule in depth.
Determine the electron geometry eg and molecular geometrymg of BCl3. 1 Answer Humaam H. Boron sits in the center of the molecule and has three valence electrons so it balances out the three chlorides.
Chemistry Molecular Orbital Theory Molecular Geometry. The Correct Answer is 120 degreesFor the molecules in which there are no lone pairs of electrons on the central atom the electronic geometry is the same as the molecular geometry. Determine the electron geometry eg and molecular geometry mg of BCl3.
There are two lone pairs around oxygen which make up the last two electron groups. Determine the electron geometry eg and molecular geometry mg of BCl3 eg trigonal bipyramidal mg tetrahedral eg trigonal planar mg trigonal planar eg trigonal planar mg tetrahedral eg tetrahedral mg trigonal pyramidal. BCl 3 Molecular Geometry And Bond Angles.
What is the value of the bond angles in BCl3. D egtrigonal planar mgbent. Draw the most appropriate Lewis structure s for BCl3.
BCl3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm. There are two O-F single bonds which makes 2 electron groups. Both BCl3 and ICl3 have 3 bonds.
What are the rules of valence shell electron pair repulsion VSEPR. Egtrigonal bipyramidal mg trigonal bipyramidal egtetrahedral mgtrigonal pyramidal egtetrahedral mgtrigonal planar egtrigonal planar mgbent egtrigonal planar mgtrigonal planar. A egtrigonal planar mgtrigonal planar.
The bond angle is 120 o. Does the molecule have a dipole. C egtetrahedral mgtrigonal pyramidal.
What is the electron domain geometry and the molecular geometry. The molecular geometry of the BeCl2 molecule is The molecular geometry of the CHF3 molecule is When in the presence of a strong acid a water As shown below an electron carrier such as PCl5 has _____ electron domains and a The information carried by a DNA molecule is in _____. Draw its VSEPR and Lewis structure.
Is BCl3 Polar or Nonpolar. Describe the hybridization electron geometry molecular geometry and polarity for each and discuss similarities and differences. Therefore from the above relation 1 the total number of valence electrons in BCl 3 is given by.
A egtrigonal planar mgtrigonal planar. The VSEPR chart is attached below. Electron geometry for this is tetrahedral.
The molecular geometry of a molecule describes the three-dimensional shape of just the atoms. Total Valence Electrons in BCl 3 3 from Boron 7 x 3 from Chlorine 24 Valence Electrons Thus BCl 3 has 24 total valence electrons. If we look at the structure BCl 3 molecular geometry is trigonal planar.
In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons. What is the molecular geometry of BCl3. Let us know in the comments below which other molecules Lewis structure you would like to learn.
Jul 12 2014 Answer link. Each sp 2 hybrid orbitals will have an unpaired electron. ClF3 has T-shaped geometry.
This gives us 21 valence electrons for 3 Chlorine atoms. The central atom also has a symmetric charge around it and the molecule is non-polar. Determine the electron geometry eg and molecular geometry mg of BCl3.
Terms in this set 53 bond angle of tetrahedral. Valence Shell Electron Pair Repulsion Theory VSEPR is used to determine the shape and bond angle of a molecule.
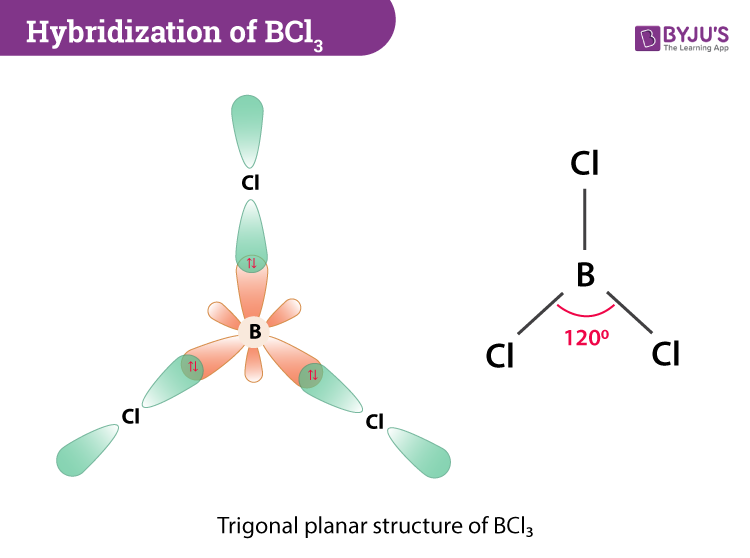
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape

Bcl3 Lewis Structure And Molecular Geometry Youtube

Bcl3 Boron Trichloride Molecular Geometry Bond Angles And Electron Geometry Youtube

Bcl3 Boron Trichloride Molecular Geometry Bond Angles And Electron Geometry Youtube
Post a Comment for "What Is The Electron Geometry Of Bcl3"